TGA Reveals Concerning Post-Market Review of Spinal Cord Stimulators
September 23, 2024
Classifying Chronic Pain
Chronic pain is a significant global health issue, recognised as a leading contributor to disability worldwide. According to data from the Global Burden of Disease study, chronic pain ranks among the top causes of years lived with disability, affecting individuals across both developed and developing nations. This type of pain not only diminishes the quality of life for those who experience it, impacting both physical and mental well-being, but it also places a considerable economic burden on society. Reduced productivity, lower workforce participation, and increased healthcare utilisation are just some of the wide-ranging societal impacts chronic pain inflicts.
The nature of chronic pain is highly complex and varied. It can stem from nociceptive conditions, where there is clear evidence of tissue damage or pathology, such as rheumatoid arthritis, or from neuropathic conditions, where the pain is caused by damage or dysfunction within the nervous system, such as in cases of diabetic neuropathy. Additionally, there are chronic pain conditions like fibromyalgia and chronic low back pain, where the link between tissue pathology and the pain experienced by the patient is less apparent. To address these ambiguities, the International Association for the Study of Pain introduced the concept of ‘nociplastic pain’ in 2016 to classify pain types that do not clearly fit into nociceptive or neuropathic categories.
In 2019, the formal classification of chronic pain in the International Classification of Diseases (ICD-11) helped further clarify its diagnosis and treatment. Chronic pain is now divided into ‘chronic primary pain’, where the pain itself is the primary condition causing disability, and ‘chronic secondary pain’, where pain is a symptom of another underlying condition. This growing understanding of the diverse causes and classifications of chronic pain highlights the need for innovative treatment approaches, particularly for those whose pain has been resistant to traditional therapies.
Spinal Cord Stimulators
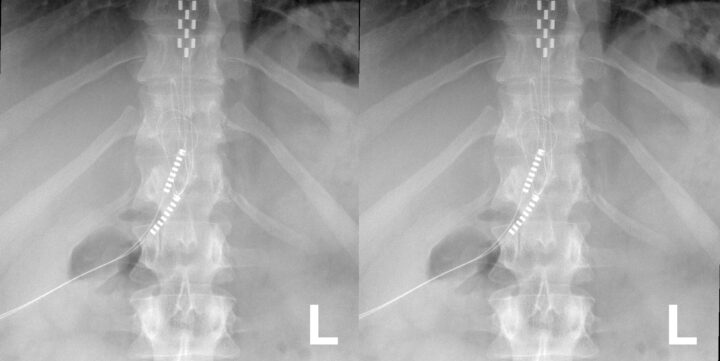
Despite advancements in understanding and classifying chronic pain, many individuals continue to struggle with pain that resists conventional treatments such as medications, physical therapy, or even surgery. For these patients, especially those dealing with severe neuropathic pain or complex regional pain syndromes, more innovative approaches are required. Spinal cord stimulation (SCS) has emerged as a promising solution for those suffering from chronic pain that does not respond to more conservative measures. This technique, which involves using electrical impulses to block pain signals from reaching the brain, represents a significant advancement in pain management, offering hope to patients for whom other treatments have failed.
Spinal cord stimulation (SCS) and dorsal root ganglion stimulation (DRGS) are forms of neuromodulation that offer targeted relief for chronic pain by altering the way pain signals are processed within the nervous system. These interventions involve the implantation of electrodes into the epidural space around the spinal cord or the dorsal root ganglion, which are connected to a pulse generator device. This device, which is either implanted under the skin or worn externally, delivers electrical impulses to the neural structures to modulate pain signals. The process typically starts with a trial phase, where temporary electrodes are used to assess the treatment’s effectiveness before permanent implantation.
The types of spinal neuromodulation devices (SNMDs) vary, not only in the specific neural targets but also in the way electrical stimulation is delivered. Conventional stimulation operates at a frequency between 40 Hz and 80 Hz, delivering a consistent pulse. More advanced methods, such as high-frequency stimulation, use a much higher frequency—up to 10 kHz—to avoid the sensation of paraesthesia (a tingling sensation often felt by patients). Burst stimulation, on the other hand, delivers intermittent trains of electrical impulses, also aiming to reduce discomfort while maintaining effective pain relief. These newer approaches, particularly high-frequency and burst stimulation, are designed to be more tolerable and may improve outcomes for patients by minimising side effects like paraesthesia.
The exact mechanisms through which neuromodulation provides pain relief are not yet fully understood, but it is believed that by electrically stimulating specific neural structures, these devices can disrupt the transmission of pain signals. This disruption may reduce the excitability of nerve cells in the affected areas, leading to reduced pain perception. Both SCS and DRGS target different parts of the nervous system and may have distinct effects, though the differences in their mechanisms and effectiveness are still being studied. Despite the ongoing debate over the optimal stimulation methods and targets, spinal cord stimulation remains an option for patients whose chronic pain has proven resistant to other treatments. An option which, in most recent times, has provided patients with an invaluable resource: hope.
Reassessment and Post-market Review
In response to growing concerns about the safety and performance of spinal cord stimulators in Australia, the Therapeutic Goods Administration (TGA) initiated a comprehensive post-market review of all SCS devices listed in the Australian Register of Therapeutic Goods (ARTG). The review, launched in 2022, aimed to assess both the safety and effectiveness of these devices based on real-world data. As part of this process, sponsors of SCS devices were required to submit detailed information, including the number of devices supplied, clinical data supporting their use, and records of adverse events or complaints.
The TGA’s investigation revealed significant areas of concern, prompting expert consultation from the Advisory Committee for Medical Devices (ACMD). The ACMD emphasised the importance of careful patient selection, noting that only a small percentage of individuals with chronic back pain progress to surgery or SCS implantation. The committee also recommended revising back pain rehabilitation guidelines to ensure that any interventions, including SCS implantation, are applied in conjunction with cause-based treatment protocols. This feedback was instrumental in shaping the TGA’s regulatory response, which included closer scrutiny of the documentation provided by manufacturers, particularly regarding instructions for use and patient information.
As a result of the review, the TGA has taken regulatory action on several SCS devices. This has included amendments to device labelling to improve clarity on risks, indications, and contraindications, as well as more detailed information on the expected lifespan of the devices. In some cases, the TGA imposed conditions of inclusion (COI) on SCS devices, requiring sponsors to meet additional post-market obligations, such as providing annual clinical follow-up studies or altering the intended purpose of the device to ensure its appropriate use. In more serious cases, some SCS devices were removed from the ARTG, preventing their use in new patients, although devices already implanted did not need to be removed from existing patients.
The TGA’s review highlights the importance of ongoing monitoring and regulation to ensure that SCS devices continue to provide safe and effective relief for chronic pain. By implementing these, the TGA aims to protect patient safety while allowing the continued use of SCS for those who can benefit from the technology.
Spinal Cord Stimulation Ongoing
Managing chronic pain remains one of the most complex challenges faced by health professionals today. The diverse causes and mechanisms of chronic pain, coupled with the variability in patient responses to treatment, make it difficult to find a one-size-fits-all solution. While spinal cord stimulators offer a promising option for patients with refractory pain, it is crucial to approach their use with caution and thorough consideration of the latest guidelines and research.
The post-market review by the Therapeutic Goods Administration highlights the importance of patient selection and the need for comprehensive rehabilitation strategies that focus on treating the underlying causes of pain. As health professionals, it is essential to critically evaluate each case individually, ensuring that the use of SCS devices is supported by robust clinical data and tailored to the patient’s specific needs.
While spinal cord stimulators represent a significant advancement in chronic pain management, their use should be guided by sound clinical judgement and adherence to best practices. By remaining vigilant and informed, we can continue to provide hope, and quantifiably improve the lives of chronic pain patients into the future.
O’Connell NE, Ferraro MC, Gibson W, Rice ASC, Vase L, Coyle D, Eccleston C. Implanted spinal neuromodulation interventions for chronic pain in adults. Cochrane Database of Systematic Reviews 2021, Issue 12. Art. No.: CD013756. DOI: 10.1002/14651858.CD013756.pub2.
Therapeutic Goods Administration (TGA). (2024, August 21). Post-market review of spinal cord stimulation (SCS) devices. https://www.tga.gov.au/how-we-regulate/supply-therapeutic-good/supply-medical-device/medical-device-post-market-reviews/post-market-review-spinal-cord-stimulation-scs-devices
Traeger AC, Gilbert SE, Harris IA, Maher CG. Spinal cord stimulation for low back pain. Cochrane Database of Systematic Reviews 2023, Issue 3. Art. No.: CD014789. DOI: 10.1002/14651858.CD014789.pub2.